 By Pasha Yuen
By Pasha Yuen
It is a year since the Human Genetic Resources (HGR) regulations were finalised, in July 2023. To celebrate this anniversary, we have created a series of blogs to examine what this regulation means to people working in clinical development.
working in clinical development.
Aiming to streamline these applications, several changes to the legislation have been made throughout the years, which now mean the review process is only 20 working days. Some of you may have heard that the HGR application process is very time consuming, but this can be improved using the tips in this series of blogs, as the regulatory review part of the process is the shortest piece!
In these series, we will walk you through the journey of HGR, its importance to clinical development, explain how to determine which samples contain genetic information, and provide insights and guidance on how to comply with this important set of regulations, allowing a swift process in applying for the HGR licence.
We start with a simple question – what is HGR?
Human Genetic Resources (HGR) regulations in China aim to protect the genetic information of the Chinese population, and to prevent this information from being exported for illegal activities.
The History of Human Genetic Resources (HGR) Regulations in China
Dating back to the 1990s, public awareness of genetic information security has been increasing amongst the Chinese population. The Ministry of Science and Technology (MOST) – a government organisation in charge of genetic resources investigation, administrative licensing, and management of clinical trials – recognised that people were starting to classify this information as sensitive personal data. Since then, several pieces of legislation were drafted by MOST to protect HGR extracted from patient samples.
Since the 1990s there have been several iterations to manage the administration of HGR, which have refined this regulation over the subsequent decades. The following timeline summarises the story of HGR regulations establishment:
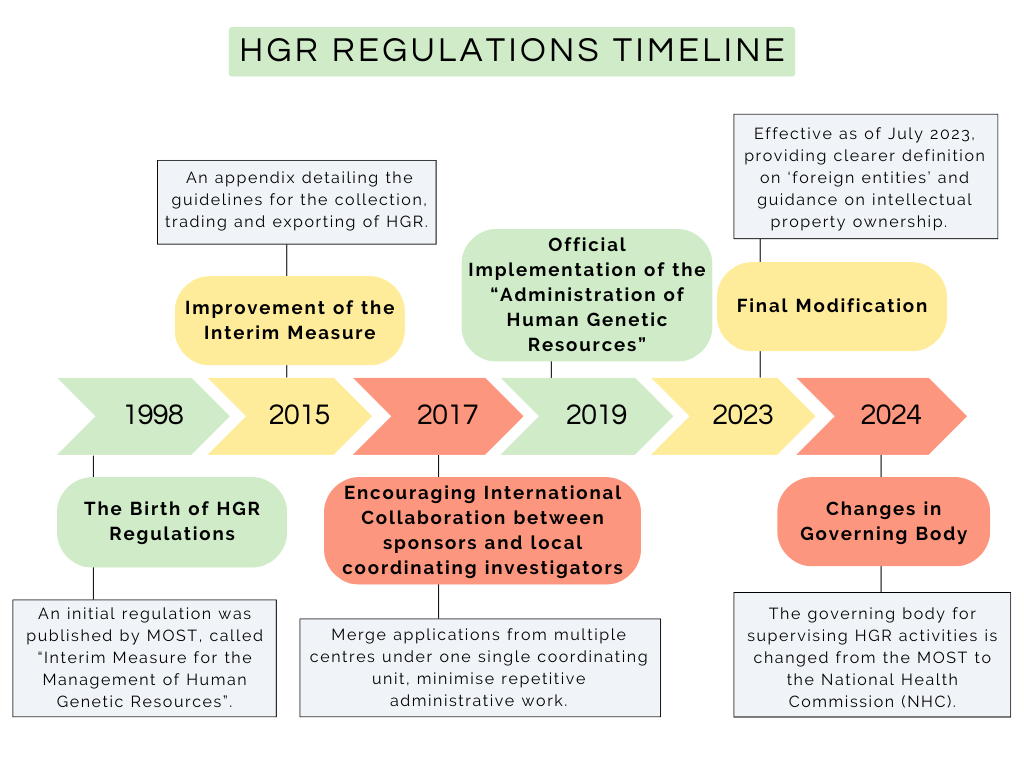 1998: The birth of HGR regulations
1998: The birth of HGR regulations
An initial regulation was published by MOST, called “Interim Measure for the Management of Human Genetic Resources”
2015: Improvement on the Interim Measure An appendix detailing the guidelines for the collection, trading and exporting of HGR
2017: Measures that encourage international sponsors to collaborate with local coordinating investigators
Merge applications from multiple centres under one single coordinating unit, minimise repetitive administrative work
2019: Official implementation of the “Administration of Human Genetic Resources”
2023: Final Modification on the legislation
Through final modification, “Implementing Rules for Human Genetic Resources Administration” was effective as of July 2023
Providing clearer definition on ‘foreign entities’ and guidance on intellectual property ownership
2024: The governing body for supervising HGR activities is changed from the MOST to the National Health Commission (NHC)
Why is this important?
When conducting clinical trials in China, patient samples will be collected, some of which will inevitably contain HGR data. Some genetic information is obvious, for example BRCA in relation to breast cancer. However, there may be genetic information in many samples that are less obvious. Almost all biological samples collected from clinical trial participants will be covered by HGR regulations, including those collected from a routine blood test. Therefore, it is important to understand when you should apply for the relevant licenses in advance of starting an international multicentre trial involving China.
The legislation is applicable to all sponsors that are not based in China, therefore it is important for all of us to have a thorough understanding of the legislation.
In the next Master Class, we will explore how to determine which samples could potentially contain human genetic information and therefore what should be reported to the relevant departments.
Learn more in Part Two