
By Srirekha Boddu
On 11th September this year Pharmora attended the EMA webinar to launch the updates to GVP Module XVI on Risk Minimisation Measures (RMM) and its Addendum II.

These updates include:
- New terminology: RMM messages and tools
- Clarification on categorisation of RMM and tools
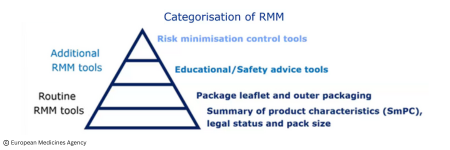
- Description of educational/safety advice tools
- Detailed guidance on Risk minimisation control tools such as:
- Defining the Healthcare professional qualification required for prescribing, dispensing and/or administration of the medicinal product
- Accreditation of the Healthcare facility
- Traceability systems
- System for documented exchange of patient information
- Certification checks for medical interventions required for prescribing or dispensing of the medicinal product
The agency discussed points to consider for selecting additional RMM tools, or adapting existing RMM, and encouraged engagement of patient and healthcare professional representatives in developing these.

This updated guidance is applicable to all new applications for marketing authorization in the EU, and for new RMM and new studies evaluating RMM. Be aware that if an existing RMM is amended, this revised guidance should be taken into account, but for now this does not apply to ongoing RMM activities.
Module XVI Addendum II – Methods for evaluating effectiveness of risk minimisation measures
EMA discussed the addendum on methods for evaluating RMM effectiveness, including:
- Scope of RMM effectiveness studies
- Schedule of RMM effectiveness PASS
- Objectives of RMM effectiveness evaluation
- Qualitative methods
- Quantitative methods such as
- Cross sectional survey
- Drug utilisation study
- Before/after interrupted time series study (they held up Time-series analysis as a gold standard)
- Mixed methods approach
They concluded that evaluating RMM effectiveness requires a multi-disciplinary approach with a combination of qualitative and quantitative methods that measure not only RMM dissemination but also risk knowledge, behaviour and health outcomes as a result of the measures.
It was great to hear directly from the agency about the latest updates to this important module.
Get in touch if you would like to find out how Pharmora can help you with RMM!