 By Dr Stephanie-Jayne Jones
By Dr Stephanie-Jayne Jones
Click here if you have missed the 1st Pharmora RMP Masterclass
 Risk management plans (RMPs) have evolved since the legislation was implemented in the EU in 2012 mandating an EU-RMP for every new licence application. At that time I had just founded Pharmora, so the general panic that ensued was obviously good for business, as pharma companies suddenly needed to create many of these documents, part of which would be published for all to see! Pharmora helped companies develop their own templates and guidance documents, and we continue to support with specialist RMP expertise.
Risk management plans (RMPs) have evolved since the legislation was implemented in the EU in 2012 mandating an EU-RMP for every new licence application. At that time I had just founded Pharmora, so the general panic that ensued was obviously good for business, as pharma companies suddenly needed to create many of these documents, part of which would be published for all to see! Pharmora helped companies develop their own templates and guidance documents, and we continue to support with specialist RMP expertise.
There are a few stumbling blocks for those developing new medicines when they begin to craft their first RMP for a novel product. Here are some tips to help avoid the biggest pitfalls:
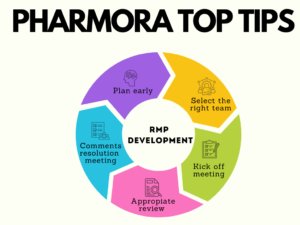
Plan early
Many pharma companies find it useful to create a “Development RMP” to support the clinical development plan, and to provide a good basis for their company core RMP ready for submission of a New Drug Application (NDA) or Marketing Authorisation Application (MAA). Not only does this save time at the end of Phase III, it can help to refine safety data collection during clinical trials so that the risks can be better characterised and managed.
Assemble the right writing team
Some companies pull anyone who had anything to do with the product into all of the meetings to discuss RMPs. There can be 50 people in these calls. This has obvious disadvantages. In reality the ideal team to lead this process consists of:
- the person writing the document (could be the safety scientist or a medical writer)
- someone with expertise in RMPs
- someone with expertise in the product and risks
it is important to be clear about the remit of the people involved. The people outlined above will be leading the writing of the document while others will be making decisions such as which risks to include and whether or not this product needs additional risk minimisation measures (aRMM).
Kick off meeting
Once the writing team have established what they need from other subject matter experts (SMEs) they should organise a kick off meeting (KOM). The purpose of this meeting should be really clearly outlined ahead of time, and only the key stakeholders should be invited.
Critical content of the KOM includes:
- assigning an accountable person for delivery of each component
- determining the timelines
- identifying any issues for discussion and who needs to make the final decision
Importantly, if the key people are unable to attend they should know that they need to nominate a deputy empowered to speak for that department, as they will be committing to timelines and deliverables at the meeting. The person sending the invite needs to chase up those people who did not accept.
At this meeting the gaps (and how they will be filled) should also be discussed. For example, if Phase III clinical trial data are awaited to inform the key risks and aRMMs, the decision-making that follows needs to be incorporated in the planned timelines.
Appropriate review
At the KOM the key reviewers and decision-makers should have been determined. If an SME wishes to solicit review from others in their department they may be able to consolidate all feedback to give to the person writing the document, or they may need the writer to do this.
In an ideal situation one SME should deliver the review on behalf of each function. In reality this is not always possible and the writer sometimes needs to consolidate conflicting and inconsistent comments from people with the same role.
Tip: to avoid too many time-consuming comments determine who the key people are in advance. If a small core team reviews and optimises the wording of the document before it is sent to the wider team for review, this can often spare the time of senior people from making the same comments or finding a conflicting way to address an issue.
Comments resolution meeting
In the situation where comments cannot be easily resolved within the document it is important to discuss these in a comments resolution meeting (CRM).
Make sure the decision-makers are at the meeting, as well as the SMEs who can provide details when the seniors ask for information. Careful consideration should be given to the attendee list to avoid inviting too many people or missing someone who has the answers to questions that will come up.
These meetings could be used for decision-making on key risks and RMMs, or that could be held outside of the writing meetings. It is important to establish the purpose of the CRM: is it for writing the document or is it to make a decision on the risks/other content? There may be a completely different set of people required for the 2 different types of meeting, and it is important for attendees understand the remit of their attendance.
Content
Once they have all been decided, an accurate and appropriate description of the risks and risk minimisation measures (RMM) is of critical importance. However, it is likely that the same wording may already exist for other products with the same risks. Obviously, the benefit-risk profile of the individual product needs to be considered, but using wording that has already been approved improves consistency for communication of specific risks across the organisation, in addition to saving time.
If you do not have any prior RMPs (e.g. many Biotech companies), or have a new risk or RMM to describe, it is important to seek help from someone with this expertise. RMP wording has a very structured approach; what you believe to be additional risk minimisation may in fact be considered routine by the regulatory agencies.
So in summary:
- Plan early;
- Assemble the right writing team;
- Hold a kick off meeting;
- Solicit appropriate review;
- Use comments resolution meetings efficiently;
- Re-use appropriate content or gain expert help where needed.
This “Top Tips” is part of our Master Class in RMPs. Check the website for a more focused description of additional risk minimisation measures, feeding back on a recent EMA webinar.
Feel free to get in touch if you would like to discuss how Pharmora could support you with RMPs.
Click here for the 3rd Pharmora RMP Masterclass